COMPASS Pathways plc announces financial results and business highlights for the Q1 2021
Highlights:
- New England Journal of Medicine publishes exploratory study showing signals of positive activity in COMP360 psilocybin compared with escitalopram for major depressive disorder
- Two further US patents granted
- Equity financing raises gross proceeds of $144 million
- Phase IIb clinical trial of COMP360 psilocybin therapy for treatment-resistant depression (TRD) on track to report data by end of 2021
- Wayne J Riley MD joins Board of Directors and Anne Benedict is appointed Chief People Officer
- Conference call today at 1:00pm UK (8:00am ET)
COMPASS Pathways plc (NASDAQ: CMPS), a mental health care company dedicated to accelerating patient access to evidence-based innovation in mental health, today reported its financial results for the first quarter 2021 and gave an update on recent progress across its business.
Our recent financing gives us additional resources to work even faster and to expand our efforts, grow our team, and focus on developing new indications, new compounds and new technologies, building on our leadership position in psilocybin therapy and mental health care. Far too many people are suffering with mental health challenges today. We are focused on developing evidence-based therapies that can make a difference and be accessible to as many patients as possible who might benefit. The COMP360 data published in the New England Journal of Medicine showed promising signals in a small investigator-initiated study. We are approaching the completion of our phase IIb trial of COMP360 psilocybin therapy for treatment-resistant depression, and on track to report data by the end of the year.
George Goldsmith, Chairman, CEO and Co-founder, COMPASS Pathways
Business highlights
- Phase IIb clinical trial of COMP360 psilocybin therapy for TRD continues to progress
- On track to report top-line data by end of 2021
- On track to report top-line data by end of 2021
- COMP360 data published in the New England Journal of Medicine
- Signal-generating, exploratory research from independent study at Imperial College London (n=59) comparing the efficacy and mechanisms of action of psilocybin with a six-week course of escitalopram, a selective serotonin reuptake inhibitor (SSRI), for major depressive disorder (MDD)
- Study showed signals of positive activity in COMP360 psilocybin when compared with escitalopram and concludes that psilocybin findings should be explored further in larger studies
- COMP360 psilocybin was generally well-tolerated and there were no Serious Adverse Events
- Signal-generating, exploratory research from independent study at Imperial College London (n=59) comparing the efficacy and mechanisms of action of psilocybin with a six-week course of escitalopram, a selective serotonin reuptake inhibitor (SSRI), for major depressive disorder (MDD)
- Two new patents granted by the US Patent and Trademark Office
- Patents cover oral formulations of COMPASS’s synthetic psilocybin in the treatment of MDD and COMPASS’s high-purity crystalline psilocybin (including the form used in COMP360), pharmaceutical formulations containing crystalline psilocybin and methods of treating MDD with crystalline psilocybin
- COMPASS’s innovation has now been recognised with six granted patents, including three in the US, two in the UK, and one in Germany
- Patents cover oral formulations of COMPASS’s synthetic psilocybin in the treatment of MDD and COMPASS’s high-purity crystalline psilocybin (including the form used in COMP360), pharmaceutical formulations containing crystalline psilocybin and methods of treating MDD with crystalline psilocybin
- Senior appointments
- Wayne J Riley MD joins COMPASS’s Board of Directors. Wayne is President of the State University of New York Downstate Health Sciences University, Brooklyn, where he holds tenured professorships in internal medicine and health policy and management. He has a breadth of experience in clinical and academic medicine, research programme oversight, biotechnology, primary care, public health, healthcare management and policy, healthcare quality, academic health science centre administration, and government service
- Anne Benedict is appointed COMPASS’s first Chief People Officer. Anne has more than 25 years of global experience in human resources, talent and organisational development, and will help COMPASS attract, retain and develop the talent and people we need to achieve our mission
- Wayne J Riley MD joins COMPASS’s Board of Directors. Wayne is President of the State University of New York Downstate Health Sciences University, Brooklyn, where he holds tenured professorships in internal medicine and health policy and management. He has a breadth of experience in clinical and academic medicine, research programme oversight, biotechnology, primary care, public health, healthcare management and policy, healthcare quality, academic health science centre administration, and government service
- Equity financing priced, raising gross proceeds of $144 million
Financial highlights
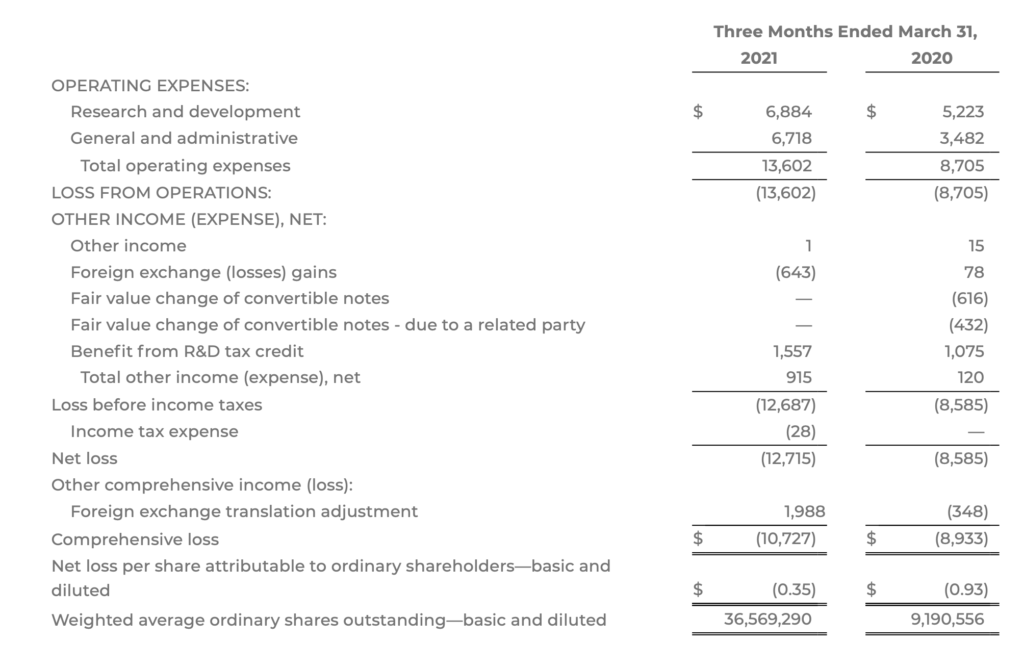
- Net loss for the three months ended March 31, 2021, was $12.7 million, or $0.35 loss per share (after including non-cash share-based compensation expense of $1.7 million), compared with $8.6 million, or $0.93 loss per share, during the same period in 2020 (after including non-cash share-based compensation expense of $1.7 million)
- Research & development (R&D) expenses were $6.9 million for the three months ended March 31, 2021, compared with $5.2 million during the same period in 2020. Of this increase, $1.4 million reflected increased development activities, including hiring additional staff, as COMPASS progresses its COMP360 psilocybin therapy in TRD, and continues to explore additional indications and therapeutic approaches
- General and administrative (G&A) expenses were $6.7 million for the three months ended March 31, 2021, compared with $3.5 million during the same period in 2020. Of the increase, $1.4 million was related to increased personnel costs, $1.1 million related to additional facility and other administrative expenses, and $0.6 million related to increased legal and professional expenses
- Pro-forma cash and cash equivalents was $179.5 million as of March 31, 2021, compared with $190.3 million at December 31, 2020. On May 4, 2021, following the end of the reporting period, COMPASS completed a public offering of 4,000,000 American Depositary Shares (“ADSs”) at a price of $36.00 per ADS for total gross proceeds of $144 million.

COMPASS PATHWAYS PLC Consolidated Balance Sheets ![]()
COMPASS PATHWAYS PLC Consolidated Statements of Operations and Comprehensive Loss
Conference call
COMPASS Pathways’ management team will host a conference call at 1.00pm UK (8.00am ET) on 13 May 2021. The call can be accessed by dialling (833) 665-0659 from the United States, +1 (914) 987-7313 internationally, and 0800 028 8438 from the UK, followed by the conference ID: 4498819.
The call will also be webcast on the investors section of the COMPASS Pathways website (ir.compasspathways.com) and archived for 30 days.
To view the original press release in its entirety click here