
PharmaDrug Provides Research Results and Initiates IND-Enabling Studies for Cepharanthine in the Treatment of Multiple Cancers
- Results of the 4 cancer types where cepharanthine provides exceptional potency and/or synergistic inhibitory effects when combined with standard of care (SoC) chemotherapy provides support for next development stage
- Advancing Cepharanthine (PD-001) to IND-enabling efficacy studies to support potential FDA clinical studies in 2022
PharmaDrug Inc.(CNSX: PHRX) (OTCMKTS: LMLLF), a specialty pharmaceutical company focused on the research, development and commercialization of controlled-substances and natural medicines such as psychedelics, cannabis and naturally-derived approved drugs, is pleased to announce positive research results for their preclinical cancer study which evaluated the effectiveness of cepharanthine-2HCl alone, or when used in combination with standard of care (SoC) chemotherapy on four undisclosed cancers. The Company is now focused on advancing to IND-enabling studies to support future FDA clinical studies in 2022.
Further to the Company’s research update on its large in vitro cancer screen studies, the Company had previously identified a short list of 23 cancers that were highly responsive to cepharanthine-2HCl. Four instances of drug synergy (cepharanthine+chemotherapy) were revealed in the latest drug combination study. Cancer cell types and standard of care (SoC) treatments remain confidential for the purpose of filing subsequent intellectual property, but here the company provides results for the four most promising types of cancer tested. The inhibitory concentration of cepharanthine (CEPN) as well as experimental positive control required to kill fifty percent of the cells (IC50) is displayed below. Lower IC50 values represent greater potency of a drug for a particular cancer. Cepharanthine displays favorable IC50 values against multiple cancer types and its safety profile in humans is supported by multiple decades of human use in Japan for alternate indications. As noted in the table below, esophageal cancer was approximately 5-times more responsive to cepharanthine than the experimental positive control; a clinically approved chemotherapeutic agent.
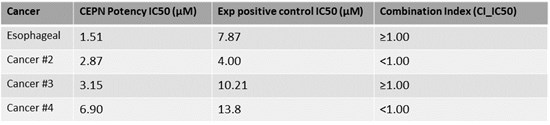
Combination index (CI_IC50) of less than 1.00 demonstrates drug combination synergy. Combination index values were generated from co-application of CEPN and a relevant clinical SoC agent for each cancer type. The experimental positive control used for in vitro studies is an approved chemotherapeutic agent.
To view an enhanced version of this graphic, please visit:
https://orders.newsfilecorp.com/files/6479/104152_5ae9f0dc44687a6d_001full.jpg
The Company has shipped its drug product, PD-001 to the clinical research organization (CRO) in support of the upcoming IND-enabling animal studies. These studies are designed to evaluate PD-001 efficacy, alone and in combination with SoC in two animal cancer models. The Company’s prime cancer focus continues to be esophageal cancer for several reasons previously stated including the orphan drug designation awarded by the FDA earlier this year. The Company has also selected a second cancer type to pursue for these studies based on multiple considerations including cepharanthine potency, ability of cepharanthine to provide synergistic benefits with SoC drugs, relative market size/need and the suitability of available animal models to provide high translation value to the program. In vitro cancer cell models, while quite useful for screening cancer types responsive to a given drug, are not ideally suited to assess a particular drug’s benefit in overcoming adaptive chemoresistance. The currently designed animal models aim to more thoroughly tackle the serious clinical issue of chemoresistance. PharmaDrug will disclose additional positive findings for the preceding 2 in vitro cancer studies once its patent council has had an opportunity to review all of the recently generated data and file a provisional patent by the end of this year. The Company also expects to publish the results in a prominent scientific journal in the near future.
We are extremely excited about the most recent research results and the potential of cepharanthine to treat various cancers alone, and in combination with SoC drugs. Moving our research from in vitro (cell culture-based models) into accepted animal models of cancer is a critical step along our path to the clinic. Careful study design has allowed us to reveal non-obvious situations where cepharanthine provides synergistic benefit to existing treatment. These findings will be leveraged to pursue new intellectual property protection and to hopefully provide new treatment options for patients suffering from difficult to treat cancers. Our move toward IND-enabling animal efficacy studies to support future human clinical studies in oncology will be commencing as planned in November.
Daniel Cohen, CEO of PharmaDrug
Rational Use of Cepharanthine to Treat Cancer
PharmaDrug’s cancer program is based on cepharanthine’s known anti-cancer activities. Cepharanthine has been shown in multiple preclinical efficacy models to inhibit cancer cell proliferation, induce cancer cell apoptosis (death) and restore cancer cell sensitivity to multiple unrelated classes of chemotherapy. Multidrug resistance in particular, continues to represent a considerable clinical challenge. As such, preclinical cancer studies aimed at elucidating the mechanisms that underly chemoresistance; including the critical role drug efflux pumps play in this phenomenon by reducing the intracellular concentration of chemotherapeutic drugs, are of particular interest to PharmaDrug. Cepharanthine has been shown in preclinical studies to potently reverse chemoresistance by downregulating expression of ABCB1, the transcript of which codes for multidrug resistance protein 1, (MDR1, aka P- glycoprotein). Importantly, several prior in vitro and in vivo studies have shown that cepharanthine-mediated reductions in ABCB1 expression restores cancer cell sensitivity to a range of chemotherapeutics including taxanes, vinca alkaloids and platinum-based drugs1-4. Collectively the studies currently being undertaken by the Company aim to identify and provide focus to novel opportunities in oncology by revealing optimal drug combinations and situations where PD-001 can prevent, lessen, or reverse chemoresistance, and/or provide additive or synergistic benefit to existing treatments. PharmaDrug’s planned animal efficacy studies, designed around the outcome of the current in vitro study, are most ideally suited to experimentally examine the role of cepharanthine in restoring chemosensitivity.
About PD-001 (Enteric-coated Cepharanthine)
Cepharanthine is a natural product and an approved drug used for more than 70 years in Japan to successfully treat a variety of acute and chronic diseases. In clinical research, Cepharanthine has been shown to exhibit multiple pharmacological properties including anti-oxidative, anti-inflammatory, immuno-regulatory, anti-cancer, anti-viral and anti-parasitic effects5,6. However, historically cepharanthine’s low oral bioavailability has represented a major obstacle to realizing its full clinical potential.
The Company is focused on advancing the clinical development of an improved oral formulation of cepharanthine (PD-001) to treat rare cancers and infectious diseases. Compared to generic cepharanthine, PD-001 has been shown in rodent and non-rodent models to possess markedly superior bioavailability (more easily absorbed). These findings support the development of an orally administered formulation, and in so doing, removes the undesirable requirement for frequent intravenous dosing.
To view the original press release in its entirety click here



