With progress made on the rare cannabinoid side of the business in 2022. InMed Pharmaceuticals Inc. (Nasdaq: INM) is re-focusing on its roots. The company is hoping for a material leap forward in its pharmaceutical pipeline in 2023, lead by advancement prospects of its lead cannabinoid-based compound for a debilitating skin condition.
Epidermolysis bullosa (EB) is a rare disease that causes fragile, blistering skin. The blisters may appear in response to minor injury, even from heat, rubbing or scratching. In severe cases, the blisters may occur inside the body, such as the lining of the mouth or stomach. Compounding the problem, the afflicted are prone to infections from open sores that never seem to heal properly.
The disease is inherited condition, usually showing up in infants or young children—although some people don’t develop symptoms until they’re teens or young adults. Epidermolysis bullosa has no cure, but mild forms may improve with age. Treatment focuses on caring for blisters and preventing new ones, which is a tough task for skin that tears easily due to genetic mutations.
InMed Pharmaceuticals’ ultimate aim is to develop a standard of care cream that can control the disease better than anything on the market. A market, we might add, that lacks an effective long term treatment option.
Speaking on the company’s focus on its most advanced program—Phase 2a clinical trial compound INM-755 for the treatment of Epidermolysis bullosa—Mr. Adams notes that as an irreversible genetic disease, symptom treatment is the goal.
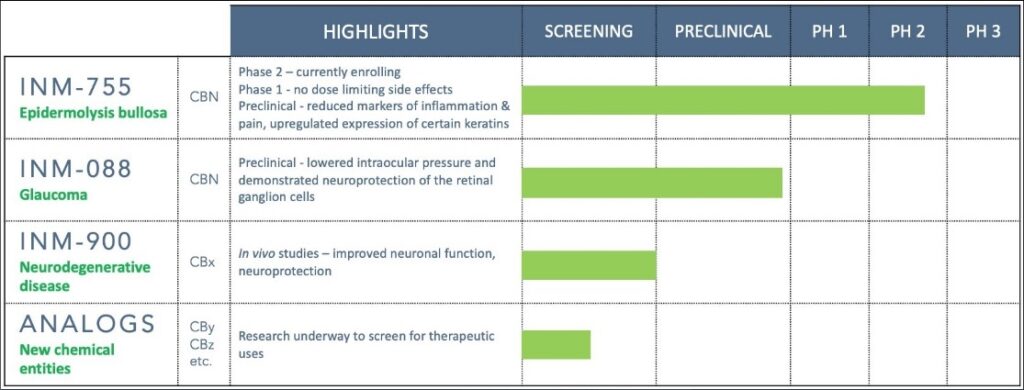
INM-755 is a topical cream with the active cannabinoid cannabinol (CBN) as its primary therapeutic ingredient. Preclinical data has already demonstrated that INM-755 may help relieve hallmark EB symptoms, such as inflammation and pain, as well potentially restore the integrity of the skin in a subset of EB Simplex patients. Phase 1 data has previously demonstrated no dose-limiting effects.
Next up in the Phase 2a portion of the trial that will determine the safe and effective dose range for the proposed therapeutic indication of INM-755. Assuming this stage clears, it will be onto Phase 2b, or human clinical trial conducted on a sufficient number of patients on which is to make a qualitative determination of efficacy and safety.
Click on the embedded link to see more of our newest interview with InMed Pharmaceuticals CEO Eric Adams, in his own words.



